- ✔ Module #1
- ✔ Module #2
- ✔ Module #3
- ✔ Module #4
- ✔ Module #5
- ✔ Module #6
- ✔ Module #7
Genset Starting Education Module #3
Solutions to Leading Causes of Battery Failure in Gensets
William F Kaewert | SENS – Stored Energy Systems LLC
The Leading Causes of Battery Failure are Improper Charging and Lack of Maintenance that Aggravate Lead-Acid Battery Failure Mechanisms1
If properly cared for, a lead-acid starting battery can perform for several years. If not cared for, however, it will fail in as little as a few months. And while the cost to replace the battery might be inexpensive, the opportunity cost of a failed genset might be measured in lost lives or financial damage. Two facts are essential in understanding genset system failures: (1) lead-acid batteries suffer from well understood failure mechanisms; (2) accurate battery charging2 dramatically slows down these failure mechanisms and can extend maintenance intervals.
Lead-Acid Battery Failure Mechanisms
Because the most commonly used energy storage device for genset starting is the lead-acid battery, it is essential that the reader understand how it fails. The primary lead-acid battery failure mechanisms are internal corrosion, sulfation and loss of electrolyte. Deeper and/or more frequent discharges accelerate these failure mechanisms, as does higher temperature.
Corrosion: The positive grid corrodes over time as lead metal of the grid oxidizes, gradually turning from metallic lead into lead dioxide (PbO2). As the grid corrodes, the positive plate grows in size because the corrosion product occupies 1.3 times the volume of the original lead metal. Simultaneously, grid corrosion causes the grid latticework to thin or break until the plate can no longer transport current to the load. Internal corrosion can be slowed but not stopped by reducing the battery’s internal temperature, which can be achieved by installing the battery in lower temperature environment and by minimizing overcharging.
Ni-Cd batteries do not suffer from grid corrosion.
Illustration 1: The original size of positive plate versus end-of-life size of positive plate3

Sulfation: Lead sulfate is created at both the negative and positive plates during discharge. If the battery is left discharged or is undercharged for long durations this accumulation builds up as a high resistance coating. Sulfation in a flooded battery can sometimes be reversed by charging the battery at voltage in excess of the manufacturer’s normally specified maximum value. Consult your battery’s manufacturer if you wish to attempt this. Sulfation reversal in absorbed glass mat/valve regulated lead acid (AGM/VRLA) batteries is not practical because the required sulfation reversal voltage is high enough to cause outgassing through the battery’s pressure relief valves, leading to battery failure due to electrolyte loss. Therefore, sulfated AGM/VRLA batteries must be replaced. Sulfation can be reduced by ensuring the battery is recharged promptly after use, and by ensuring that the battery is charged at a high enough voltage to reverse regular sulfation.
Ni-Cd batteries do not suffer from damage due to sulfation.
Loss of electrolyte: Both flooded and AGM/VRLA batteries are subject to electrolyte loss. Hydrogen and oxygen evolve as a byproduct of charging. Gradual loss of electrolyte from flooded batteries is easily managed by periodic maintenance that includes addition of deionized water. In contrast, electrolyte lost from AGM/VRLA batteries cannot be replaced. The only protection against an AGM/VRLA battery drying out is ensuring that it is not charged at too high a voltage.
As with lead-acid batteries, Ni-Cd batteries lose electrolyte during charging and must be periodically topped up with deionized water to maintain proper electrolyte level.
Flooded Lead-Acid Battery Versus AGM/VRLA Lead-Acid Battery
The plates of most SLI batteries are immersed in liquid electrolyte that is held inside the battery by gravity. This arrangement is referred to as either a “flooded” or “vented” battery because plates are normally submerged. Oxygen and hydrogen gases liberated from the battery during recharge escape to the atmosphere through the battery vent caps. Some loss of these gases during charging is normal and is the reason deionized water must be periodically added to a flooded battery. Overcharging the battery greatly accelerates gas generation and water loss. Permanent battery damage occurs when electrolyte level is too low, allowing active material to dry out.
Some lead-acid batteries immobilize the liquid electrolyte using a technology called absorbed glass mat (AGM). Inside the AGM battery electrolyte is absorbed by a porous fiberglass separator material wrapped around the battery’s plates. This technology offers two potential advantages: (1) there is little free electrolyte available to leak if the battery is damaged, and (2) hydrogen and oxygen recombine inside the battery back into water, meaning that under ideal conditions water does not have to be added. The vent caps of AGM batteries are thus permanently fixed, meaning there is no possibility to replenish water. The AGM battery is sometimes referred to as a “maintenance-free” battery.
Illustration 2: 1000x magnification of absorbed glass mat material in AGM battery4

Cell caps on AGM batteries look like vent caps on flooded batteries but are very different in function. Each cell of an AGM must be closed to the atmosphere so that oxygen and hydrogen created during charge may recombine back into water, rather than escape the battery. The cell cap of an AGM battery is a one-way pressure relief valve set at about 5 pounds per square inch. When correctly charged the pressure relief valve does not operate and gas stays within the battery jar. When overcharged, though, gas is generated faster than it can recombine back into water. The pressure relief valve(s) then open until cell pressure drops to about 3 psi. The gas is lost for good and there is no way to replenish the correct proportions of oxygen and hydrogen. AGM batteries thus dry out each time they are overcharged. When sufficiently dry they will fail completely.
Lead-acid batteries with pressure relief valves are referred to generically as “VRLA”, for Valve Regulated Lead Acid. Because the vent caps are not removable, battery state of charge cannot be assessed by measuring electrolyte specific gravity.
Accurate Battery Charging is Essential
One of the lead-acid batteries most demanding characteristics is the need for precision charging. As shown below in illustration 3, charging at too low a voltage (undercharging) leads to battery failure due to sulfation. Charging at too high a voltage (overcharging) causes excess battery gassing, loss of electrolyte and accelerated plate corrosion. In the case of AGM type lead-acid batteries excess gassing causes failure due to battery dry-out.5 For example, overcharging an AGM battery by the apparently small voltage of 0.05 volts per cell (2.2%) can cut battery life by 50%.6 Overcharging by 0.10 (4.4%) volts per cell can cut battery life by 75%. “Proper and adequate charging is the single most important factor in obtaining optimum life from a [VRLA/AGM] battery.”7
The ideal float voltage is a delicate balance between under- and overcharging. Achieving this balance is complicated by the fact that the ideal charging voltage varies with temperature.8 When the battery is warm the correct charging voltage is lower than when the battery is cold. Illustration 3 shows that achieving the correct charging voltage as temperatures change is like threading a needle between regions of undercharge damage and overcharge damage.
Illustration 3: Temperature-compensated charging voltage in a lead-acid starting battery9
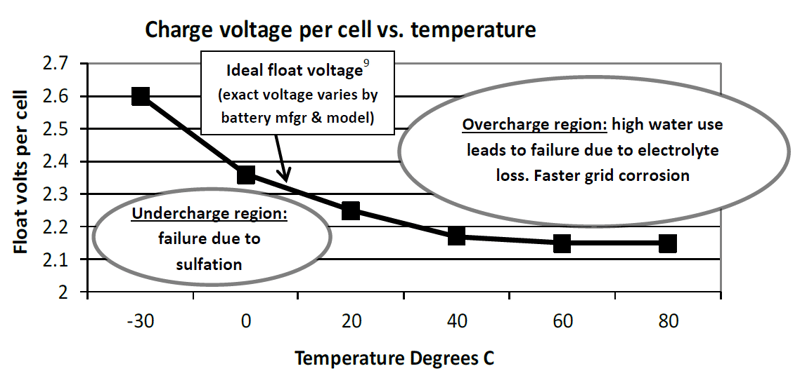
While all automotive voltage regulators vary their output with temperature, many battery chargers are not temperature compensated. This means that such chargers either over- or undercharge batteries, increasing risk of genset start failure. Because genset chargers are on 24/7 instead of a few hundred hours per year, the consequence of incorrect charging is roughly 18 times worse for a genset battery than a vehicle battery. It is up to the user or specifying engineer to demand temperature compensated charging in order to assure this important feature is provided.
The examples below demonstrate the problem of using a non-temperature compensated battery charger:
- A lead-acid battery operated at a normal summer temperature of 50 degrees C10 (122F) but charged at the manufacturer’s recommended 2.25 volts/cell at room temperature would suffer a reduction in life of more than 70%11 because it is overcharged.
- During winter in most parts of North America the same lead-acid battery could be exposed to 0 degrees C (32F) temperature or colder. If charging voltages were not increased above the room temperature value of 2.25 volts/cell to about 2.35 volts/cell, the battery would be undercharged. Undercharging would degrade the battery’s already poor cold temperature performance and increase risk of irreversible damage due to sulfation.
Basic Battery Charging Recommendations
The bare minimum battery charger performance requirements are as follows and apply to both lead-acid and nickel-cadmium batteries. The genset owner or maintainer must insure that:
- Battery charger float voltage is set to the correct value recommended by the battery manufacturer. Different battery manufacturers recommend different voltage values because they use different specific gravities of electrolyte and different formulations of grid alloy and active material.
- The battery charger is guaranteed by its manufacturer to regulate DC output voltage to ±1% or better overall AC input and load conditions. This capability is essential to maintaining the battery’s voltage in the narrow region between under- and overcharge.
- The battery charger must automatically temperature compensate its output voltage so that charging voltage tracks the ideal voltage value at the temperature at which the battery is operating. All automotive voltage regulators are temperature compensated, and some chargers are available with this essential feature. Temperature compensation must be specified at time of charger purchase.
- If a battery heater is used, the charger must be equipped with a remote battery temperature compensation option, the remote sensing option must be used, and the remote sensing probe must be physically connected to the battery’s case or terminal post. These measures are necessary to inform the charger’s temperature compensation system of the battery’s actual temperature.
Additional battery charger requirements are identified in SENS Genset Starting Education Module #5: How to Specify a Genset Battery Charger
Regular Battery Maintenance is Essential
Because they significantly reduce both over- and undercharging, properly specified chargers can safely reduce the frequency of battery preventive maintenance. In all non-AGM/VRLA batteries, however, electrolyte must be regularly maintained in order to replenish water loss due to electrolysis.12 All batteries, including AGM/VRLA, require periodic visual inspection for problems and retorquing of high current connections.
Proper maintenance can delay but not prevent battery failure. Lead-acid batteries can fail prematurely and suddenly because of improper application or charging or lack of a preventive maintenance program. Batteries can fail to provide the expected life if the specifier does not recognize and address the conditions that cause failure. While it should be noted that Ni-Cd batteries do not suddenly die and are more abuse-resistant than lead-acid types, they also must be maintained regularly.
An effective maintenance program requires a schedule for each item and a written record of results, including figures such as volts per cell or specific gravity for flooded lead-acid batteries. It is recommended that users become familiar with IEEE guidelines for the installation, testing and replacement of batteries. Any and all readings taken should be compared to the previous readings to determine if any rapid changes have occurred. Significant changes demand corrective action that may include applying an equalizing charger or replacing the battery. Maintenance tasks include, but are not limited to the following:
- Periodic water replacement is the most important maintenance function. All batteries use water.13 Battery charging current, particularly when overcharging, decomposes water into hydrogen and oxygen. Deionized or distilled water is recommended when topping up non-AGM, non-VRLA type batteries.14 Under no circumstances should the electrolyte level be allowed to drop below the top of the separator in a cell. This would reduce the battery's discharge capacity, and the battery plates could suffer permanent damage. Excessive water usage by any or all cells should be noted and the cause determined.
- Cell voltages should be measured routinely. One weak cell makes a bad battery, and weak cells tend to get weaker faster than other cells. Early detection of a weak cell enables corrective action to be taken sooner. Unfortunately, it is not possible to measure individual cell voltages inside a six-cell (12V) lead-acid battery.
- Specific gravity is a useful indicator of the state of charge of a flooded lead-acid battery. As lead batteries age, their specific gravities may drop, indicating plate sulfation which in turn is a reliable sign of reduced capacity. In some cases, low specific gravities may be remedied with a Boost or Equalize charge but only if the weak battery condition is recent rather than chronic. Investigating the root cause of the low specific gravity condition is essential.15 Specific gravity is not a useful measure of Ni-Cd battery state of charge or health.
- Battery connections, particularly lead-acid, require periodic retorquing16 because lead metal is soft and slowly deforms at room temperature under the compression of terminal bolts. Any discoloration or other evidence of heating of battery connections should be noted and remedied, as this indicates a loose connection. Ni-Cd battery terminals are made of harder metal than the terminals of lead-acid batteries and so are less affected by cold-flow problems.
- Keep the battery clean. Dirt can cause earth/ground leakage across or between cells, and batteries must be kept clean. Evidence of electrolyte leakage, overcharging, gassing, and loose terminals is quickly noticeable when the battery is clean.
- At least once per year cell voltage or specific gravity should be checked for equality. Cells that fail to charge equally or use excessive water are a sign of trouble and should be investigated or replaced.
- Proactively replace shorter-lived batteries. Proactive replacement of weak batteries avoids the inevitable time when a battery fails to crank the engine. When maintained at a moderate temperature and properly charged an AGM/VRLA type starting battery can last between 3 and 5 years. If improperly charged or operated at very high temperatures, battery lifetime could be just a small fraction of this 3-5 year “best-case” period. Ni-Cd type batteries can be expected to last 15-18 years provided that regular maintenance is performed.17
Summary of Key Points
- The leading causes of battery failure are improper charging and lack of maintenance that aggravate lead-acid battery failure mechanisms.
- The primary lead-acid battery failure mechanisms are internal corrosion, sulfation and loss of electrolyte. Deeper and/or more frequent discharges accelerate these failure mechanisms, as does installation in high temperature environments.
- The normal charging process causes some of the battery’s electrolyte to dissociate into hydrogen and oxygen. This is remedied in flooded batteries by periodically refilling them with distilled water. Overcharging greatly accelerates the rate of water usage.
- AGM/VRLA batteries cannot be refilled, meaning that there is no remedy for excess gas generated by overcharging. The battery dries out each time it is overcharged, and when it is sufficiently dry it will fail completely.
- One of the lead-acid batteries most demanding characteristics is the need for precision charging. Charging at too low a voltage (undercharging) leads to battery failure due to sulfation. Charging at too high a voltage (overcharging) causes excess battery gassing, loss of electrolyte and accelerated plate corrosion.
- The ideal charging voltage varies with temperature. Achieving the correct charging voltage as temperatures change is like threading a needle between regions of undercharge damage and overcharge damage.
- Because genset chargers are on 24/7 instead of a few hundred hours per year, the consequence of incorrect charging is roughly 18 times worse for a genset battery than a vehicle battery.
- Proper maintenance can delay but not prevent battery failure. Flooded batteries must be regularly maintained in order to replenish water loss due to electrolysis. Also essential are visual inspection for problems and retorquing of high current connections.
- An effective maintenance program requires a schedule for each item and a written record of results, including figures such as volts per cell or specific gravity for flooded lead-acid batteries.
References
- And, in the case of a Ni-Cd battery, improper commissioning.
- See SENS Genset Starting Education Module: #6: Battery Charging Basics
- East Penn Manufacturing
- East Penn Manufacturing
- The battery dries out because the outgassing of hydrogen and oxygen cannot be countered by the addition of water to battery cells. This is because AGM/VRLA battery vent caps are not removable.
- GNB; Installation/Operating instructions for Absolyte UP.
- Battery Application Handbook for Cyclon® & Genesis® Sealed-Lead Products.
- While the shape of the curve is common to all lead-acid batteries, values on the curve may vary from supplier to supplier. It is essential that users ensure that float and boost charging voltages are set to the values recommended by their battery’s manufacturer.
- Battery voltage data: Caterpillar Inc. Chart: Stored Energy Systems LLC (SENS). NOTE 1: This curve represents only one battery type and is not ideal for all batteries. NOTE 2: The relatively high voltage below 0 degrees C may not be suited to all batteries or genset electrical systems. NOTE 3: To enable compliance with battery makers’ different temperature compensation slopes, the battery charger’s temperature compensation slope should be adjustable.
- Genset underhood temperatures of 70 degrees C (158F) have been measured in generators exposed to direct sun in high ambient temperatures.
- Temperature compensated charge voltage per the chart at 50 degrees C (122 degrees F) is about 2.16 volts/cell. Failure to adjust the charge voltage down to this value would leave the battery overcharged by 2.25–2.16, or 0.09 volts/cell. This is very close to the 75% life reduction value specified by GNB for 0.1 volt/cell overcharge.
- Breakdown of electrolyte water into hydrogen and oxygen gases caused by passage of charging current through the battery.
- Including the class of batteries known as “maintenance-free”, AGM or VRLA. Under normal circumstances the hydrogen and oxygen gases liberated inside these cells during charging are recombined inside the battery back into water.
- AGM/VRLA batteries are permanently sealed, so water lost during charging cannot be replaced.
- Causes of low specific gravity include undercharging (which could potentially be remedied by boost charging), or sulfation – in which case the damage is irreversible, meaning the battery must be replaced.
- Refer to retorquing intervals and torque values provided by the battery’s manufacturer.
- Table 2 in SENS Genset Starting Education Module #2: Engine Start Battery Performance Characteristics shows typical battery life versus temperature.
