- ✔ Module #1
- ✔ Module #2
- ✔ Module #3
- ✔ Module #4
- ✔ Module #5
- ✔ Module #6
- ✔ Module #7
Genset Starting Education Module #2
Engine Start Battery Performance Characteristics
William F Kaewert | SENS – Stored Energy Systems LLC
Understanding Batteries: Key Concepts and Definitions
Overcome unexpected power drops – reliable battery performance keeps gensets ready when it matters most.
Batteries are unique chemical devices that function differently - and often less predictably - than the electrical, electronic, and mechanical systems typically found in a genset.
- Battery Basics: An electric storage battery is made up of interconnected electrochemical cells that provide DC current. The battery’s nominal voltage depends on the number of cells connected in series:
- Lead-acid cells have a nominal voltage of 2.0 volts, so six lead-acid cells in series create a 12-volt battery.1
- Nickel-cadmium (Ni-Cd) cells have a nominal voltage of 1.2 volts, requiring ten cells in series for a 12-volt battery.
- Energy and Discharge Power Rate: Due to electrochemical reaction kinetics2 and diffusion characteristics3, batteries deliver less total energy as discharge power rate increases. This means:
- Batteries provide their full rated ampere-hour (AH) capacity only when discharged slowly.
- For rapid discharges, such as during engine starts, batteries deliver a smaller fraction of their total capacity before the voltage drops below a useful level.
- Voltage Requirements for Engine Start: It is critical to size the battery so that even under worst-case conditions, its voltage remains above a minimum level to keep the engine’s control computer powered. Table 1 below4 provides typical minimum allowable battery voltages for engine starting applications.
Table 1: Typical minimum allowable DC voltages during engine cranking
| Break-away period (first second during crank) | Rolling current | |
|---|---|---|
12-volt lead-acid |
6.0 volts |
9.0 volts |
24-volt lead-acid |
12.0 volts |
18.0 volts |
Battery Performance Degrades at Cold Temperatures
Batteries struggle to deliver full power in cold conditions, risking unexpected failure.
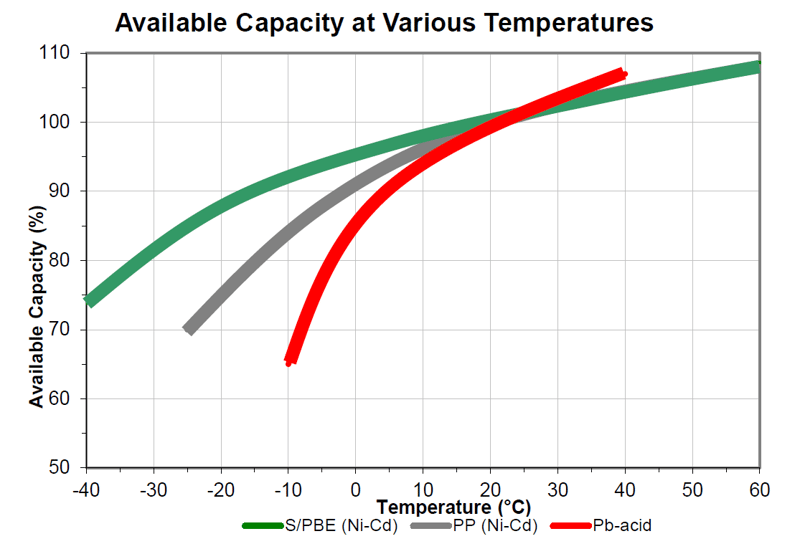
All batteries experience reduced performance at lower temperatures due to slower chemical reactions within the battery. For instance, a lead-acid starting battery rated to deliver 1,805 CCA (Cold Cranking Amps) at 32°F (0°C) will only deliver 1,444 CCA at 0°F (-18°C). Typically, a lead-acid battery derates by about 1.1% per degree Celsius (0.6% per degree Fahrenheit)5. Practical solutions to compensate for this performance drop include oversizing or heating the battery. Although Ni-Cd batteries also degrade in cold conditions, they retain functionality at lower temperatures and derate less than lead-acid batteries, as shown in the illustration below.
- Oversizing the Battery
Lead-acid batteries are relatively inexpensive, making oversizing a simple and cost-effective solution. While Ni-Cd batteries also require derating in cold conditions, they generally degrade less than lead-acid types. For specific battery sizing and the correct battery for your worst-case cold temperature, consult your battery supplier. - Using Battery Heaters
Thermostatically controlled, AC-powered blanket heaters can warm the battery, reducing or even eliminating the need for cold temperature derating. However, heating the battery places specific demands on the charging system, which, if not applied correctly, can lead to battery failure. If using a battery heater, the charger should have a remote temperature compensation system with a probe directly attached to the battery. Failing to use a remote temperature probe risks overcharging, which could reduce battery life by up to 75%6. Only purpose-built heaters should be used, and as of now, there are no UL-listed heating systems available for Ni-Cd batteries. - Using a Ni-Cd Battery
Pocket plate, PBE, and fiber electrode type Ni-Cd batteries experience less cold temperature performance loss compared to lead-acid batteries7, as shown in Illustration 1 below. However, Ni-Cd batteries also suffer performance losses in cold environments and may still require temperature management.
Note: For best results when using battery heaters, remote battery temperature compensation is essential to prevent overcharging. Incorrect application could cause significant battery degradation, impacting performance and longevity. Refer to Illustration 3 in Genset Starting Education Module #3: Solutions to Leading Causes of Battery Failure in Gensets for additional guidance on temperature compensation.
Illustration 1: Different derating with temperature of different types of batteries
Impact of High Temperatures on Battery Longevity
High temperatures significantly reduces battery life, increasing the risk of costly, premature replacement.
Battery life decreases as the battery’s average yearly temperature increases above room temperature. Each eight degree C (14.4F) temperature increase above 25 degrees C (77F) cuts lead-acid battery life in half. Corresponding loss of Ni-Cd life is 18%. The chart below compares battery typical initial expected life to life expectations at different temperatures.
Table 2: Life derating versus temperature lead-acid and Ni-Cd batteries
| Avg. Temp (degrees F) | Lead-acid battery life | Ni-Cd battery life |
|---|---|---|
77 |
4 years8 |
20 years |
91 |
2 years |
16.4 years |
106 |
1 years |
13.5 years |
120 |
Six months |
11 years |
135 |
< 3 months |
9 years |
Ni-Cd batteries are obviously a better choice for very high and very cold temperature applications.
There is no life “credit” for operation at cold temperatures. Batteries exposed to extreme heat in summer and extreme cold in winter will lose life when hot but not regain it when cold.
Summary of Key Points
- Batteries are chemical devices that behave differently from, and less predictably than, the electrical, electronic and mechanical systems most common in a genset.
- Battery performance degrades at cold temperatures. Lead-acid batteries suffer a steeper fall-off in performance with temperature than do nickel-cadmium batteries.
- When battery blanket heaters are used in cold climates, the battery charger must be equipped with a remote temperature compensation system, and the remote temperature compensation probe must be attached to the heated battery.
- Battery life is reduced at high temperatures. Lead-acid technology suffers worse life loss with temperature than does nickel-cadmium.
References
- Nominal" voltage is the open-circuit voltage of a battery that is in a charged condition. During charging the charging source's voltage must be higher than nominal to enable current to flow from the charger into the battery.
- The rate of an electrochemical process. Many things determine this rate, including temperature, specific gravity of electrolyte and quality of the interface between the battery's electrode and electrolyte.
- The rate at which electrons move through the battery's materials and across boundaries between materials.
- EGSA 100b, Recommended Practice for Engine Cranking Batteries Used with Engine Generator Sets.
- Rolls Surrette Battery.
- Refer to Illustration 3 in SENS Genset Starting Education Module #3: Solutions to Leading Causes of Battery Failure in Gensets. At 0 degrees C the output of a temperature compensated battery without remote temperature probe could be as high as 2.36 volts. Although this would be the correct charging value for the battery shown in the illustration, it would be 0.16 volts higher than the correct voltage of 2.20 volts/cell for a battery heated to 30 degrees C, or 86 F. Using a remote temperature compensation probe is essential to prevent overcharging when using a battery heater.
- EGSA Electrical Start Systems training course.
- Typical life under ideal conditions of temperature, charging, use, etc. Actual in-service life will be shorter under real-world conditions.
